-
Graduation
Certificate
-
ECTS-Points
24
-
Learning format
-
Duration
2 semesters, part time
-
Costs
EUR 7.500,--
-
Admission requirements
Bachelor's degree and professional experience
-
Language
English
-
Study location
Krems (AT)
-
Curriculum
-
-


Assoz.-Prof. Dr.
Jens Hartmann
Course Director - Department for Biomedical Research
Questions about the study course?
Please feel free to contact us directly.
Your Benefits of our Program
- Current challenges such as the disruption of supply chains or process changes due to big data and digitalization will be addressed.
- Working in small groups offers the opportunity to directly transfer the knowledge and experience of our lecturers - all of whom are international experts in their field.
- Graduates of the program are part of a network of successful innovators and entrepreneurs in their field.
-
networking
-
trending topic
-
international
Stackable Program
In combination with other continuing education programs, Stackable Programs can be combined to achieve an academic degree.
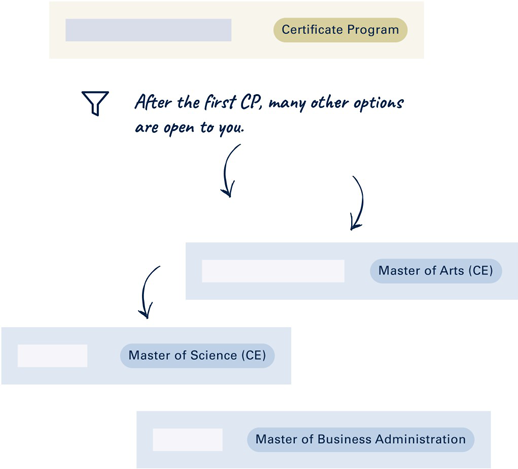

The Stackable Program offers me complete flexibility in the design of my studies.
Tatjana Kohl
Student
Curriculum
The Certificate Program Biotech, Pharma & MedTech Management prepares you for the special features of the strictly regulated healthcare market and its competitive international environment. It consists of four modules of four days each in blended learning format.
-
Contents
- Health Care Markets I
- Health Care Markets II
- Compliance & Governance
- Supply Chain Management
-
Contents
- Quality Management & Lean Management
- Intellectual Properties Management
- Regulatory Affairs
- GMP/GLP/GDP/GCP
-
Contents
- Digitalization in Health Care
- Big Data Management and Precision Medicine
- R&D Strategies
- Technology Transfer
-
Contents
- Digitalization in Health Care
- Big Data Management and Precision Medicine
- R&D Strategies
- Technology Transfer
Study Program Director
Downloads
Here you find further informationen about the Certificate Program „Biotech, Pharma & MedTech Management“.
Application
Admission Requirements
- Austrian degree (bachelor or higher) or equivalent degree of a foreign university and
- a minimum of two years professional experience in the field
- English Knowledge (C1)
Application Process
-
Contact us to clarify your expectations of the Certificate Program and fulfilment of the admission requirements
-
Online application (enter study identification number UM 992 578 in the online tool)
-
Enrolment procedure (interview with the course director)
-
Admission to the program
We look forward to receiving your application and to welcoming you soon at the University for Continuing Education Krems.
Stackable Studies & Programs
Tags


